Energy by nuclear fusion of hydrogen

The Sun is a main-sequence star, and thus generates its energy by nuclear fusion of hydrogen nuclei into helium. In its core, the Sun fuses 620 million metric tons of hydrogen each second.
This blog will guide you. how easy the physics is. and give many information around the world about physics. I guaranteed, you will be interesting in physics.







 The fusion of two nuclei with lower masses than iron (which, along with nickel, has the largest binding energy per nucleon) generally releases energy, while the fusion of nuclei heavier than iron absorbs energy. The opposite is true for the reverse process, nuclear fission.
This means that fusion generally occurs for lighter elements only, and
likewise, that fission normally occurs only for heavier elements. There
are extreme astrophysical events that can lead to short periods of fusion with heavier nuclei. This is the process that gives rise to nucleosynthesis, the creation of the heavy elements during events such as supernovae.
The fusion of two nuclei with lower masses than iron (which, along with nickel, has the largest binding energy per nucleon) generally releases energy, while the fusion of nuclei heavier than iron absorbs energy. The opposite is true for the reverse process, nuclear fission.
This means that fusion generally occurs for lighter elements only, and
likewise, that fission normally occurs only for heavier elements. There
are extreme astrophysical events that can lead to short periods of fusion with heavier nuclei. This is the process that gives rise to nucleosynthesis, the creation of the heavy elements during events such as supernovae. Fission as encountered in the modern world is usually a deliberately-produced man-made nuclear reaction induced by a neutron. It is less commonly encountered as a natural form of spontaneous radioactive decay
(not requiring a neutron), occurring especially in very
high-mass-number isotopes. The unpredictable composition of the products
(which vary in a broad probabilistic and somewhat chaotic manner)
distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time.
Fission as encountered in the modern world is usually a deliberately-produced man-made nuclear reaction induced by a neutron. It is less commonly encountered as a natural form of spontaneous radioactive decay
(not requiring a neutron), occurring especially in very
high-mass-number isotopes. The unpredictable composition of the products
(which vary in a broad probabilistic and somewhat chaotic manner)
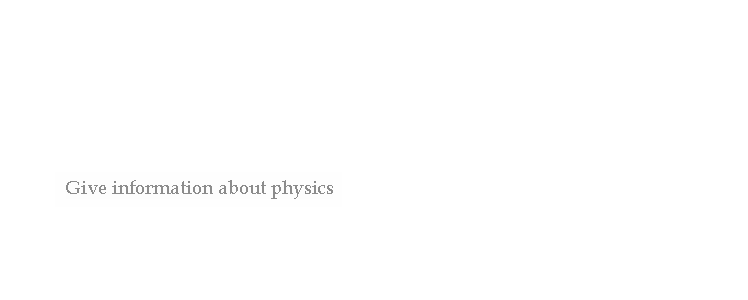
distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time.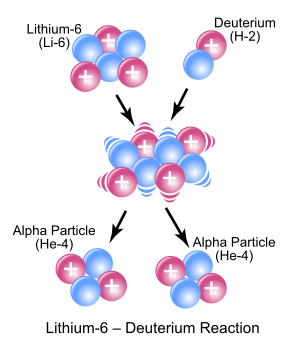 In principle, a reaction can involve more than two particles
colliding, but because the probability of three or more nuclei to meet
at the same time at the same place is much less than for two nuclei,
such an event is exceptionally rare (see triple alpha process for an example very close to a three-body nuclear reaction). "Nuclear reaction" is a term implying an induced change in a nuclide, and thus it does not apply to any type of radioactive decay (which by definition is a spontaneous process).
In principle, a reaction can involve more than two particles
colliding, but because the probability of three or more nuclei to meet
at the same time at the same place is much less than for two nuclei,
such an event is exceptionally rare (see triple alpha process for an example very close to a three-body nuclear reaction). "Nuclear reaction" is a term implying an induced change in a nuclide, and thus it does not apply to any type of radioactive decay (which by definition is a spontaneous process).